License Out
Licensing of Our Pipeline
As a drug discovery bio-venture, we aim to out-license our pipeline under development (several pipelines have already advanced to Phase II clinical trials) to pharmaceutical companies as much as possible. In addition to licensing out, we are also flexible to cooperate in other forms of collaboration, such as joint development.
We would like to briefly introduce our pipeline. Please contact us if you would like us to send you materials or meet with you for a more detailed explanation.
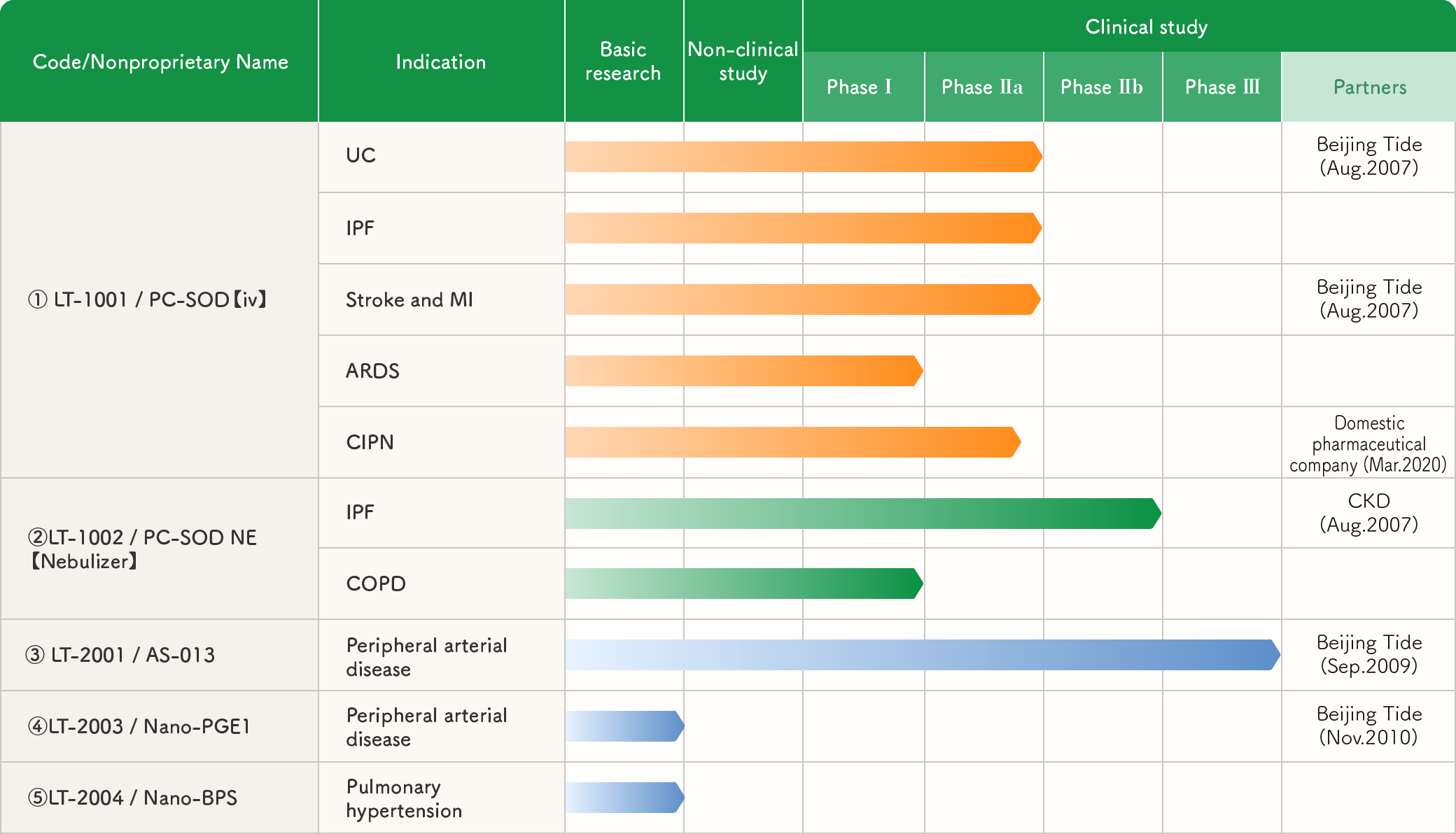
Major pipelines and their status (DDS-related)
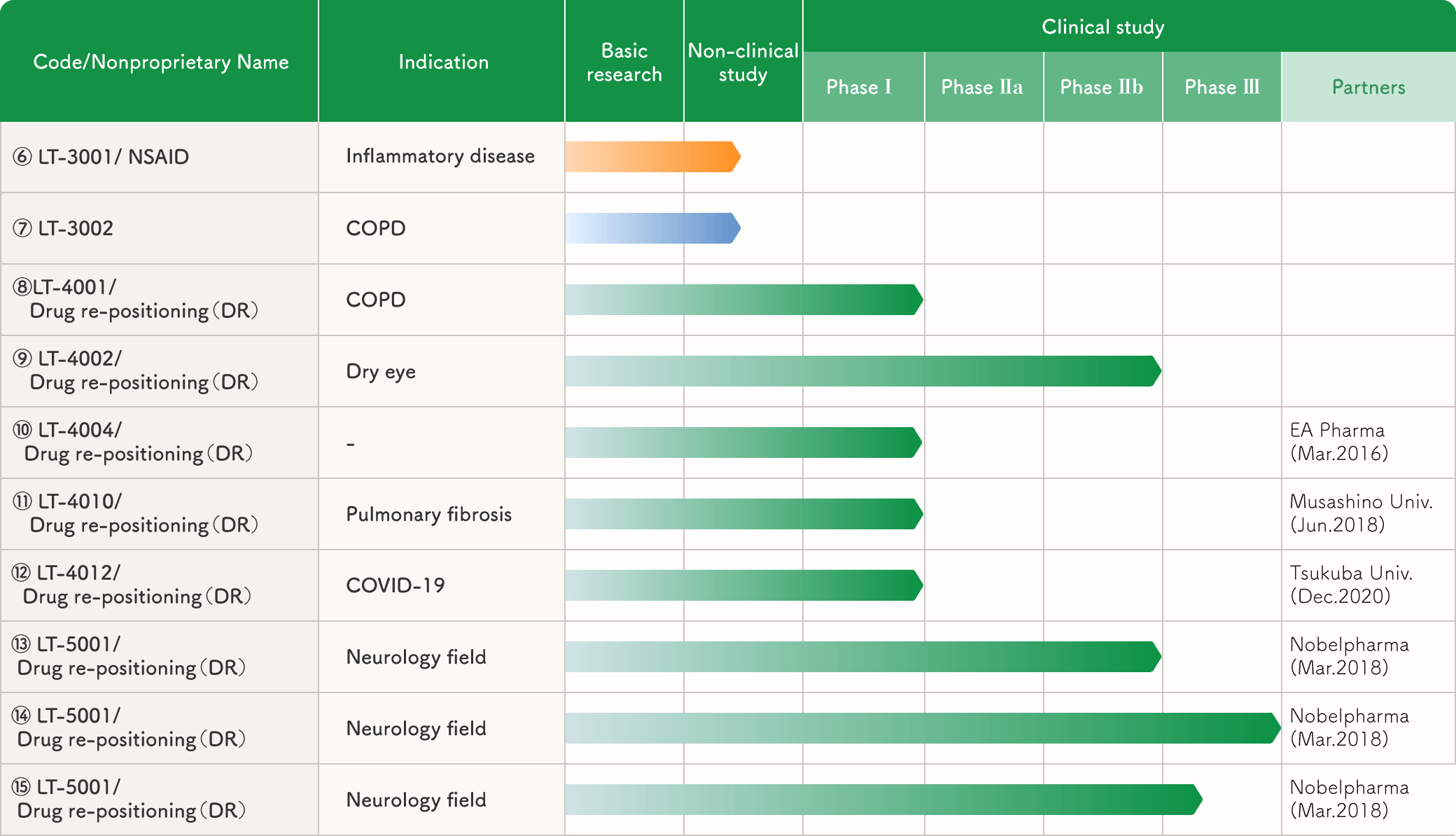
Major pipelines and their status (DR-related)
(1)(2) PC-SOD as a therapeutic agent for CIPN, myocardial infarction, renal disease, and ARDS
【Target Diseases】CIPN, myocardial infarction, renal failure, ARDS, etc.
【Development Stage】completed Phase II clinical trials
【Target Drug】LT-1001 (injection), LT-1002 (inhalation) (PC-SOD)
【Intellectual Property】Substance patents, use patents, and formulation patents
PC-SOD, which effectively scavenges reactive oxygen species, the root cause of many diseases, is a promising therapeutic agent for a variety of ailments.
In fact, our clinical trials have shown efficacy in CIPN, idiopathic pulmonary fibrosis and ulcerative colitis. In China, we are currently conducting phase II clinical trials for ischemia-reperfusion injury after myocardial infarction.
CIPN is a type of side effect associated with anticancer drug therapy, in which numbness and other symptoms occur after anticancer drug administration, and in severe cases, treatment such as discontinuation or reduction of the dose of anticancer drugs is required, thus preventing the continuation of cancer treatment itself. This is a major problem in clinical practice. We have discovered through animal experiments that PC-SOD has a preventive effect, and we have completed Phase II clinical trial. In animal models, PC-SOD has been shown to be effective in a wide range of diseases, including COPD, cerebral infarction, spinal cord injury, burns, traumatic brain injury, transplantation injury, myocardial infarction, renal disease, and ARDS. For any of these diseases, we can license our substance and use patents (for some diseases).
(3) DDS carrier stealth-type nanoparticles with both targeting and sustained release properties
While previous DDS carriers aimed for either targeting or sustained release, we have succeeded for the first time in the world in developing stealth-type nanoparticles that achieve both at the same time. The technology described in (4) and (5) below is based on this technology, but we also hold a patent on the particles themselves, so we can conduct joint research and development to incorporate your desired drug product into these particles.
(4) Nano-PGE1 as a treatment for peripheral arterial occlusive disease
【Target Diseases】Peripheral arterial occlusive disease
【Development Stage】Non-clinical studies are underway.
【Target Drug】LT-2003 (nano PGE1)
【Intellectual Property】Formulation patents
Lipo-PGE1, developed by our company, has contributed to the treatment of many patients. However, it requires daily injections, which is problematic in terms of quality of life. Therefore, we developed nano PGE1, which is PGE1 encapsulated in DDS carrier stealth-type nanoparticles with both accumulation and sustained release properties. This formulation is expected to be administered once every two weeks and to be more effective than daily administration of lipo-PGE1.
(5) Nano-PGI2 derivative as a therapeutic agent for pulmonary hypertension
【Target Diseases】Pulmonary hypertension
【Development Stage】Non-clinical studies are underway.
【Target Drug】LT-2004 (nano PGI2 derivative)
【Intellectual Property】Formulation patents
Currently, pulmonary hypertension is treated by continuous administration of PGI2 by pump or oral administration of PGI2 derivatives, but the former is problematic in terms of quality of life and the latter in terms of efficacy. We have therefore developed a nano PGI2 derivative encapsulated in a DDS carrier stealth-type nanoparticle that has both accumulation and sustained release properties. This formulation accumulates in the vascular lesion and releases the PGI2 derivative in a sustained manner, so it is expected to be sufficiently effective even when administered once every two weeks.
(6) A new NSAID that is less likely to cause gastric ulcers and is fast-acting
【Target Diseases】Inflammatory diseases
【Development Stage】Non-clinical studies are underway.
【Target Drug】LT-3001 (new substance)
【Intellectual Property】Substance patents
Non-steroidal anti-inflammatory drugs (NSAIDs) are clinically essential as antipyretic, analgesic, and anti-inflammatory drugs, but gastric ulcer side effects are a major problem. We have discovered a new NSAID (LT-3001) that is much less likely to cause gastric ulcer than existing NSAIDs and exhibits analgesic effects more rapidly.
(7) COPD drug with both long-acting bronchodilator and anti-inflammatory effects (new substance)
【Target Diseases】COPD
【Development Stage】Non-clinical studies are underway.
【Target Drug】LT-3002 (new substance)
【Intellectual Property】Substance patents
Currently, COPD treatment involves both long-acting bronchodilators to improve symptoms and steroids to inhibit disease progression. In contrast, LT-3002, which we discovered in animal experiments, not only dilates the bronchi longer than existing bronchodilators, but also exerts stronger anti-inflammatory effects than steroids. We hope to license out this new substance, which is very promising as a COPD treatment, from the preclinical stage.
(8) COPD drug with both bronchodilator and anti-inflammatory effects (already approved drug for another indication)
【Target Diseases】COPD
【Development Stage】Since the drug is already approved, non-clinical and phase I clinical trials have been completed.
【Target Drug】LT-4001 (already approved drug for another indication)
【Intellectual Property】Use patents and formulation patents
Currently, COPD treatment involves both long-acting bronchodilators to improve symptoms and steroids to inhibit disease progression. In response, we have discovered LT-4001, an approved drug that combines bronchodilator and anti-inflammatory effects, from our library of approved drugs.
(9) Dry eye treatment with a new mechanism
(already approved drug for another indication)
【Target Diseases】Dry eye
【Development Stage】Completed late phase II clinical trials
【Target Drug】LT-4002 (already approved)
【Intellectual Property】Use patents and formulation patents
Although drugs with various mechanisms have been marketed and developed for dry eye, no treatment has yet been established. Noting that there are currently no drugs that protect the cornea from damage caused by hyperosmolarity of tear fluid, we searched our library of approved drugs for such substances and discovered LT-4002, an approved drug. Subsequently, we conducted early phase II clinical trials, which suggested its safety and efficacy. Next, we conducted a late phase II clinical trial. The results showed a trend toward symptom improvement, but the targeted significant difference could not be achieved. Therefore, we are looking for a partner to conduct Phase III and late Phase II clinical trials in collaboration with us.
(11) Drug for pulmonary fibrosis with a new mechanism
(already approved drug for another indication)
【Target Diseases】Pulmonary fibrosis
【Development Stage】Since the drug is already approved, non-clinical and phase I clinical trials have been completed.
【Target Drug】LT-4010 (already approved drug for another indication)
【Intellectual Property】Use Patents
Idiopathic pulmonary fibrosis is a disease in which the lungs gradually become fibrotic and respiratory function declines, and the prognosis is said to be worse than lung cancer with a five-year survival rate of less than 40%. It is believed that myofibroblast activation is the cause of this disease. Therefore, in collaboration with Musashino University, we screened the approved drug library for drugs that suppress myofibroblast activity and discovered the approved drug LT-4010.
(12) COVID-19 infection treatment with a new mechanism of action
(already approved drug for another indication)
【Target Diseases】COVID-19 infection
【Development Stage】Since the drug is already approved, non-clinical and phase I clinical trials have been completed.
【Target Drug】LT-4012 (already approved drug for another indication)
【Intellectual Property】Use Patents
From the perspective of contributing to society, we also worked on COVID-19 infections. Specifically, we conducted screening in collaboration with the University of Tsukuba Faculty of Medicine and discovered LT-4012, an already approved drug. This drug suppresses the growth of novel coronaviruses through a new mechanism, and has been effective in almost completely suppressing viral growth in vitro. It has also been shown to suppress virus-dependent individual mouse deaths in animal experiments.