Contract Research/Consultation
DR joint research and development, and DR contract research
In order to efficiently conduct DR, we have been conducting research based on the strategy of building our own chemical library of approved drugs, which consists of more than 1,000 of the approximately 1,200 approved drugs on the market in Japan and USA, screening those with the desired pharmacological effects from the library, and confirming their effects in clinical trials. For example, after discovering an approved drug effective for dry eyes from the library and filing a patent application for its use, we were able to promptly start phase II clinical trials (usefulness of DR). On the other hand, there are an increasing number of cases where pharmaceutical companies wish to discover an approved drug that is effective for a specific disease or acts by a specific mechanism and conduct DR, or where pharmaceutical companies wish to discover a new indication for an approved drug or discontinued product and conduct DR. We provide materials (such as libraries of approved drugs) and know-how (effective drug selection, intellectual property strategies, drug pricing strategies, etc.) accumulated through our DR research to date, and conduct joint DR research and development as well as DR contract research.
Case Studies
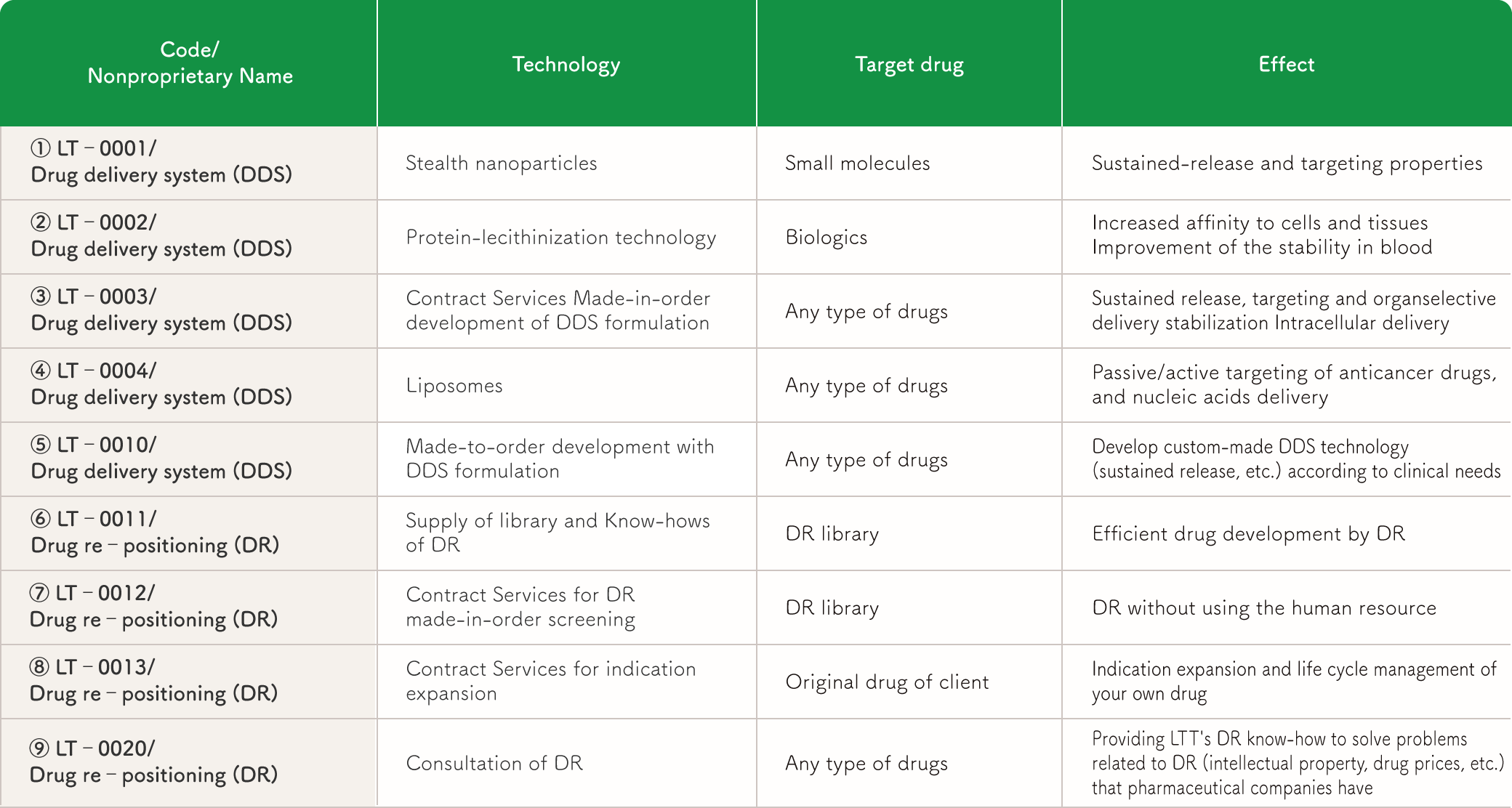
DR Joint Research and Development (LT-0011)
Companies with screening systems or research resources for screening are eligible for this program. We will provide a library of approved drugs free of charge, as well as the know-how and materials obtained from its past experience in narrowing down drug candidates, conducting research necessary to bridge the gap to the clinic, and conducting research necessary for intellectual property rights. Our laboratory can also perform some of this research. If an interesting candidate drug is obtained, we would ask you to file a patent application for it jointly with us, and in the case of DR, we would file a usage patent, a dosage and administration patent, and a formulation patent, etc. By utilizing our experience and know-how in filing numerous patents in DR, we believe that effective patent applications will be possible. We can play a leading role in the preparation for clinical trials, including manufacturing of the drug product, preparation of clinical trial protocols, and PMDA compliance.
DR Screening Contract Services (LT-0012)
We undertake research to screen already-approved drugs with specific pharmacological effects. In such cases, we proceed with the research in the same manner as described above, apply for a use patent, formulation patent, and substance patent, and assign them to the contractor. In some cases, we can even conduct POC clinical trials and assign the results as well (this is possible because of our extensive experience in conducting clinical trials related to DR). The advantage of this contract research is that the company can discover an approved drug with the desired pharmacological effect without having to devote its own human resources.
Commissioned project for expansion of indications (LT-0013)
We are contracted to discover new indications for drugs already on the market, new drugs in development, or drugs that are no longer in development. In such cases, we perform various types of drug re-profiling (including subcontracting), discover new indications, file application for use patents, and assign them to the contracted companies. In particular, if we succeed in discovering and developing a new indication for a new drug whose development has been halted due to lack of efficacy, we believe that this would be very beneficial because you would not have to waste the money you have spent so far.
Collaborative research and development of DDS products, contracted development of DDS products
As a pioneer in drug delivery system (DDS) research, we have experience in launching DDS new drugs and are still developing them. On the other hand, many pharmaceutical companies are downsizing their formulation development (including DDS) divisions and accelerating outsourcing in order to improve R&D efficiency. In response to this trend, we will provide tailor-made formulation development and DDS technology development, making full use of the formulation development (DDS) technologies that we have cultivated to date, as described below. Many companies are contracted to develop drug formulations, but many of them manufacture products using methods specified by the client company or using the company’s own formulation technology as is. In contrast, we utilize our experience in formulation studies to establish a formulation from scratch in order to achieve the pharmacokinetics requested by the client company. We will do our best to meet the client’s needs, whether it is joint development or contracted development, so please feel free to contact us. We also work with contracted academia for contract research.
Case Study
Solubilization of insoluble API (LT-0003), (LT-0004)
We have formulation technologies such as liposomes and emulsions, and are particularly good at solubilization and PK improvement of poorly soluble APIs. We are especially good at solubilization and PK improvement of poorly soluble APIs. As a result, we have achieved the target pharmacokinetics of liposome encapsulation of poorly soluble compounds with our original formulation under contract research from a pharmaceutical company and provided samples for animal experiments.
Biodegradable polymer compound, polylactic acid (PLA), for formulation (LT-0001)
We also specialize in formulations using PLA, a biodegradable polymer compound. For example, we have established and obtained intellectual property for stealth-type nanoparticles (nanoparticles with a size of about 100 nm) by encapsulating a charged or hydrophobic low-molecular-weight compound in PLA nanoparticles and coating the surface with PEG. In the case of highly fat-soluble compounds, it is also possible to adjust the release rate by encapsulating the API in an oil layer together with a specific polymer, and to prepare an emulsion formulation with a low initial burst. We have already succeeded in encapsulating nucleic acids in these particles. This technology has been developed in collaboration with Professor Tsutomu Ishihara, College of Engineering, Nihon University.
Improvement of PK of Biopharmaceuticals by Phosphatidylcholine (PC) Modification (LT-0002)
PEGylation is a well-known technology for improving biopharmaceuticals for the purpose of PK improvement. On the other hand, we have phosphatidylcholine (PC) modification technology, which is our original technology in the world. By modifying proteins and enzymes with PC, we have succeeded in increasing their affinity for cell surfaces while improving their blood retention. This is a major difference from PEG, which only improves blood retention but decreases affinity to cells. We are conducting clinical trials using a formulation that applies this PC-modification (PC-SOD, which is PC-modified superoxide dismutase that has the ability to remove reactive oxygen species). Furthermore, we are also developing an improved biopharmaceutical (Biobetter) by modifying a biopharmaceutical already on the market with PC, which we believe will enable a reduction in dosage due to improved pharmacokinetics. This PC modification technology can improve the kinetics and efficacy of not only proteins but also peptides.
Development of new DDS technology to meet the needs (LT-0010)
One of the characteristics of our DDS technology is that it is disease oriented rather than technology oriented. In other words, we have developed DDS technologies such as lecithinization in response to the need for such DDS technologies to treat diseases. On the other hand, as mentioned above, we have various DDS technologies, and we also collaborate with academia that has various DDS technologies. Therefore, if we receive a need for “development of such DDS technology for this API (sustained release, targeting, improvement of blood retention, etc.),” we will consider developing a new tailor-made DDS technology.
DR Consulting Business
Drug repositioning (DR) is widely accepted worldwide as an efficient drug discovery method. On the other hand, non-scientific issues often hinder the success of DR.
(1) Older drugs are much cheaper due to drug price reductions and are not profitable at those prices. How can we obtain higher drug prices?
(2) How can a use patent, which is the lifeline of DR, be granted?
(3) What should be done to prevent generic entry under the use patent?
(4) In the case of older drugs, toxicity tests, etc., are conducted under old standards, but do they need to be redone?
(5) Can an application for approval be filed without the cooperation of the original manufacturer?
I believe that in many cases, it is not possible to promote DR that is beneficial to patients due to problems such as the following. As a pioneer in DR, Dr. Tohru Mizushima, president of our company, has consulted with many companies. Therefore, we have launched a DR consulting business to help resolve such problems, and as a first step, we have concluded a contract with ASKA Pharmaceutical Holdings Co., Ltd. We would like to make use of our know-how gained from our long years of DR experience to make your DR a success, so please do not hesitate to contact us.