DR (Drug Repositioning)
What is DR
(Drug Repositioning)?
Drug repositioning (DR) is a strategy to discover new pharmacological effects of approved drugs with well-confirmed safety and pharmacokinetics in humans and to develop them as treatments for other diseases.
Our DR features
We were the first in Japan to point out the importance of DR and have developed as a leading DR company.
Drug screening using a library of approved drugs
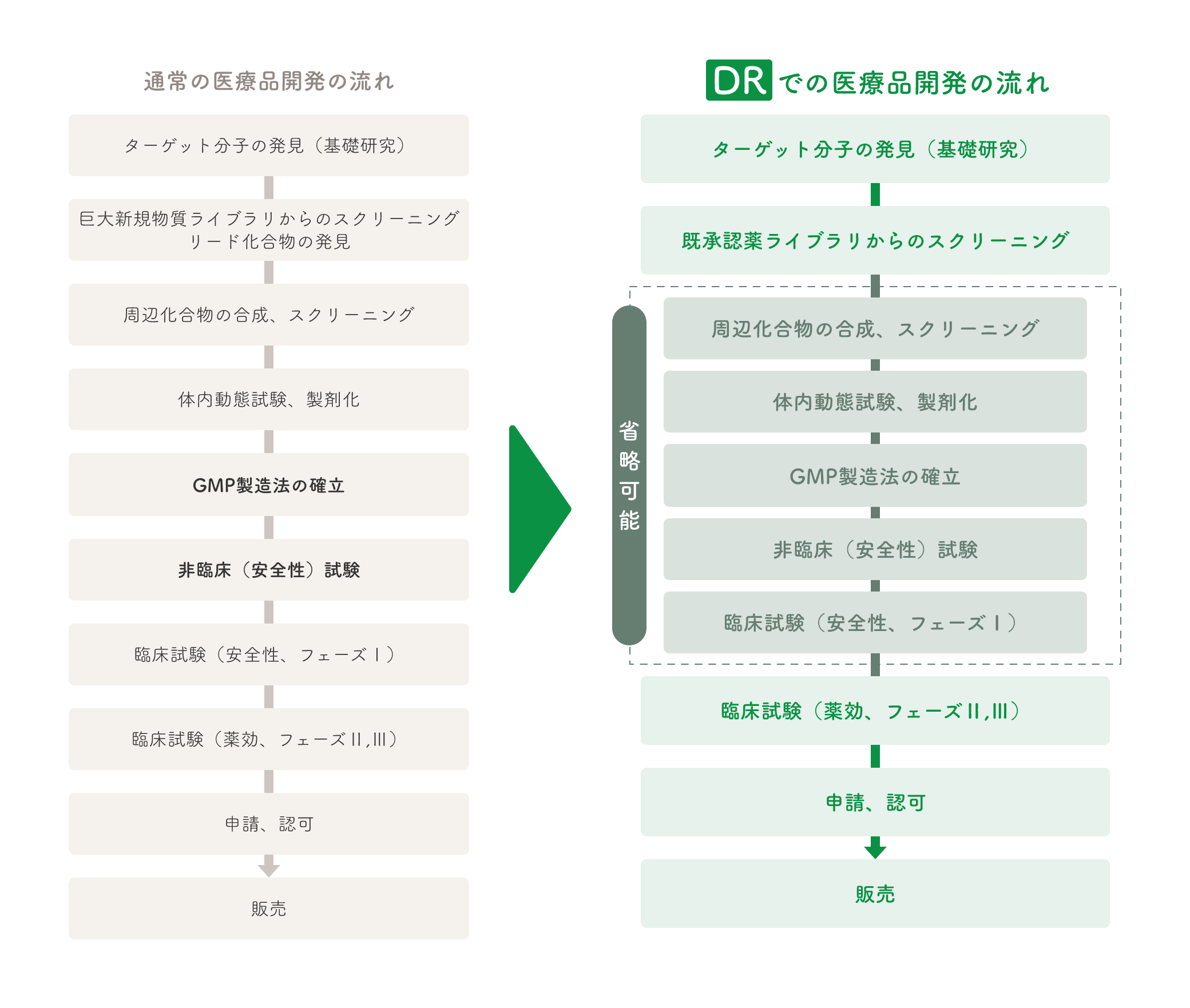
In order to promote DR efficiently, we have established our own compound library of drugs approved in Japan and the U.S. (previously approved drugs). Using this library, we have conducted various screenings to advance DR (COPD, dry eye, etc.). We also provide this library and research funds to conduct joint research with academia.
New drug development using already approved drugs as lead compounds
In order to further enhance the efficacy of approved drugs obtained through screening or to obtain substance patents, we have synthesized derivatives of approved drugsand created new substances (LT-3001, LT-3002, etc.).
Revival of discontinued products by DR
Many pharmaceutical companies have compounds that have been confirmed safe in clinical trials but whose clinical development has been suspended due to lack of efficacy or other reasons (new drugs in storage). We are also in the business of performing such DR on behalf of major companies, and have jointly filied patents.
Advantages of DR Research
The advantages of DR in drug development include

Low risk of clinical trial failure due to side effects.

By using already existing data, we can reduces development time and cost.

In silico screening and in silico clinical trials are possible.
Previous Cases

Development of COPD treatment using library of approved drugs

Development of dry eye treatment using a library of approved drugs

Development of anti-inflammatory drugs with fewer side effects using DR

Contracted by a pharmaceutical company to conduct research on the expansion of indications.

Collaboration with academia using libraries of approved drugs
DR drug development flow
DR Grant
We are developing a project to collaborate with academia by providing a library of previously approved drugs and research funding. For more information, please see this page.
Consultation
and Educational Activities
As a pioneer in DR, we are available for consultation with our experience and much expertise in DR. For more information, please see this page.
Licensing-in/
Cooperative Development
We support the development of new drugs through joint research and development agreements for companies (especially small and medium-sized enterprises and venture companies). For more information, please see this page.
Contract Research/
Consultation
As a pioneer in DR, we have a great deal of experience and know-how in DR and provide consultation services for DR projects that are in need of screening, intellectual property, and bridging to clinical trials. Please see this page for more details.